New Insights on COVID-19 Vaccine Trials: A Doctor's Perspective
Written on
Chapter 1: The Decision to Participate
As a 52-year-old board-certified infectious diseases physician, I made the choice to volunteer for the COVID-19 vaccine trial, specifically the Moderna vaccine. My background includes training at the CDC in the Epidemic Intelligence Service, often likened to the medical CIA.
I also identify as a Black woman, and my involvement in this trial aims to demonstrate to others in the Black community my belief in science and the critical role of vaccinations. Recent statistics from a survey conducted by STAT and The Harris Poll reveal a declining willingness among Americans to receive a COVID-19 vaccine, particularly among Black Americans.
Section 1.1: Conversations and Community Trust
Throughout this pandemic, I’ve engaged in discussions with over a thousand individuals, revealing a stark need for community trust and unity, which has been overshadowed by fear and skepticism.
During a conversation with a middle-aged man, I inquired about what would convince him to get vaccinated. He responded, “Seeing other Black individuals involved would help.”
A week later, I spoke with a group of seniors who expressed hesitance regarding the vaccine, largely due to its politicization. I strive to remind people that the vaccine development process is grounded in science, not politics, though I often question if they truly believe me. On the same day, I completed the eligibility questionnaire for a Phase 3 study at George Washington University.
I felt no apprehension; knowing that Phase 3 trials indicate prior safety assessments reassured me.
Subsection 1.1.1: Understanding the Research Process
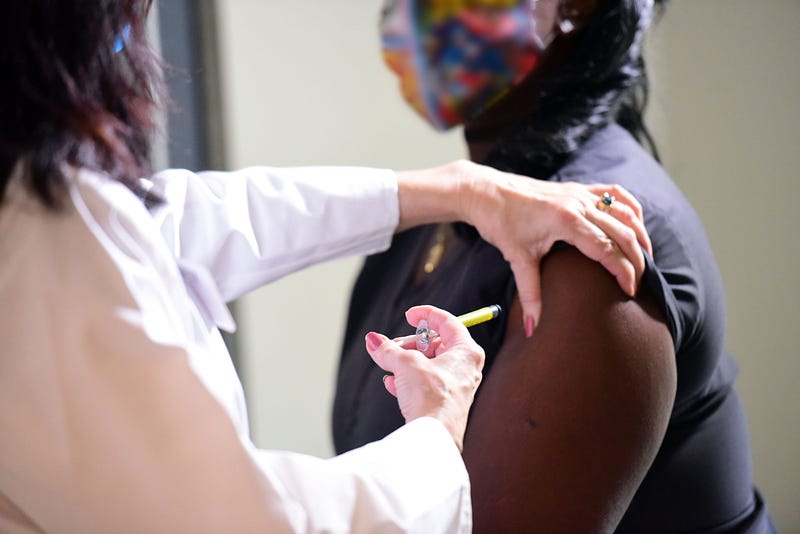
Experiencing the process firsthand is essential for educating others. Despite having received numerous vaccines, I had never participated in a research study until now. Two weeks later, I received a call confirming my participation in the Moderna vaccine trial, and my first visit took place four weeks ago.
Arriving at the infectious diseases clinic at GW felt much like a typical doctor’s appointment. The initial step involved addressing my questions and signing an informed consent form.
I emphasize this aspect to those skeptical of the research process, particularly in light of historical injustices like the Tuskegee Syphilis Study. Although that study ended in 1972, which is within my lifetime, the memory of such events understandably fosters distrust.
Nevertheless, it’s crucial to recognize the progress made since then. With safeguards like informed consent and the active involvement of Black scientists and researchers, I want to reassure people that participation is voluntary, safe, and that medical care is available should any complications arise.
Section 1.2: My Experience as a Trial Participant
Aside from attending clinic appointments, my life has remained largely unchanged, save for a few minor side effects. The logistical aspects of the trial have been seamless, with no wait times at the clinic and a consistently supportive atmosphere.
I have undergone two clinic visits and received two vaccine doses, spaced one month apart. Each visit involved monitoring my vital signs, discussing any symptoms, and answering my questions. Additionally, each visit included a nasal swab coronavirus test, which, while uncomfortable, is essential for evaluating the vaccine's efficacy.
The night after my first vaccination, I awoke with a throbbing arm pain, accompanied by body aches. After taking ibuprofen, I managed to return to sleep. By morning, the soreness had significantly decreased, and I felt fatigued but well overall. My only side effects after the second dose were slight fatigue and some arm discomfort.
In trials like this, participants have a 50% chance of receiving the actual vaccine or a placebo. Should vaccinated individuals develop COVID-19 over the next year or two, it suggests the vaccine’s ineffectiveness. Conversely, if most cases occur among those receiving the placebo, it indicates success.
Chapter 2: Moving Forward with Transparency
The challenging part of the trial is now completed; the remaining phase involves observation. Monthly check-ins with the study team allow them to assess any side effects or concerns until the study concludes or is halted based on the data.
This unpredictability is why no one can accurately predict when the vaccine will become available; it’s a natural process that must unfold over time.
I urge the public to disregard political discourse surrounding scientific matters. It baffles me why media outlets interview politicians regarding scientific processes—they lack expertise in this area, which only leads to public confusion, as we currently observe.
I am dedicated to utilizing my expertise to clarify the coronavirus and vaccine trial processes. Additionally, I am involved in a collaborative effort, the Black Coalition Against Covid (BCAC), aimed at combating misinformation within the Black community. This initiative includes virtual town hall meetings featuring Black doctors and scientists, including myself, to inform and educate our communities.
Ultimately, I believe that demonstrating my confidence in vaccine science through transparency about my experiences is the most effective way to educate others and promote participation. I invite anyone with questions or concerns to reach out to me. After all, it’s Health Literacy Month—a perfect opportunity to celebrate knowledge and understanding.
Lisa Fitzpatrick is a board-certified infectious diseases physician and medical epidemiologist. Connect with her at Lisa Fitzpatrick, MD. For more insightful articles, visit Business Insider’s homepage.